Slides
Collections
Slideboxes
My Workspace & Organizations
Shares
Courses and Quizzes
Case sharing
AI image analysis marketplace
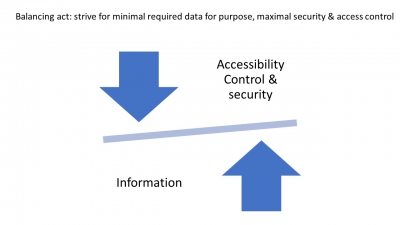
Security and data compliance
PMA.start
Names and definitions
Questions or in need of support? Contact helpdesk@pathomation.com or go to our helpdesk page
Archived pages: